|
The New Mexico Liquid Sodium α ω Dynamo Experiment SAFETY PRACTICES, PROCEDURES AND COMPLIANCEThis document is intended as the application of sodium metal safety information specific to the New Mexico Liquid Sodium α ω Dynamo Experiment. The Dynamo Project Group (DPG) wishes to present how we will institute, administrate and manage sodium metal safety practices, procedures and compliance specific to our experiment. The New Mexico Liquid Sodium α ω Dynamo Experiment will be performed in compliance with Federal, State, Industry and Institutional safety practices and procedures involving the receipt, storage, handling, and use of sodium metal. Following the introduction, the safety requirements concerning the receipt, storage, handling, fire fighting, and contingency plan for spill and cleanup are covered first. Subsequently we present how these standards will be applied to the requirements specific to the experiment. The DPG safety-officer/contact-person is Howard F. Beckley, New Mexico Institute of Mining and Technology (NMIMT), Department of Physics. He can be reached at the Dynamo Lab: (505) 835-5384, Dept. of Physics: (505) 835-5328, email: hbeckley@nmt.edu. The NMIMT Energetic Materials Research and Testing Center (EMRTC) will assign an engineer to the Liquid Sodium α ω Dynamo Experiment project to oversee that all safety practices and procedures are adhered to during operations that involve the sodium metal. The EMRTC engineer/safety-contact-person has yet to be identified. The DPG members consisting of Howard F. Beckley, Stirling A. Colgate and Van D. Romero, plus the EMRTC engineer will all have to sign off on the safety and operational readiness of the experiment. The aforementioned individuals will all be responsible for the safe operation of the New Mexico Liquid Sodium α ω Dynamo Experiment. IntroductionThe fear of sodium may have been fueled by youthful indiscretion in the chemistry lab, yet metallic sodium is a major industrial chemical. Domestic and foreign industry has used millions of tons of metallic sodium over the last 100 years. It is shipped in steel drums, ISO tanks, tank trailers and railroad tank cars of greater than 40 tons each.1 The mass of sodium in each tank car is more than several hundred times the mass of sodium used in our experiment. When sodium metal is shipped in steel drums, the container is non-returnable. The Liquid Sodium α ω Dynamo Experiment will use ~ 330 lb of sodium metal in the apparatus, shipped to us in a steel drum.2 Liquid sodium is the lowest density, high conductivity fluid that is not a biological poison. There is a vast difference between using sodium at 110 C as in this experiment and at 600 C as a fast reactor coolant. Much of the high-end technology of liquid sodium use was developed for the high temperature coolant purpose. Two facilities, built on a massive scale and now surplus, have been used for recent successful sodium dynamo experiments in East Germany and Latvia.3-5 These experiments were performed in immense halls for full containment of a liquid sodium equipment failure. We believe that this scale, cost, and the equipment is an unnecessary burden. We expect to test a different dynamo flow field in more modern and modest equipment that is developed and constructed in the laboratory, but tested at an existing local facility designed for this purpose. The experimental apparatus and related equipment for the Liquid Sodium Dynamo has been designed to be semi-portable and remotely operated. Whenever testing is to take place with liquid sodium, all the equipment will be moved to a proven testing facility for high-energy materials: The Energetic Materials Research and Testing Center (EMRTC) facility at the New Mexico Institute of Mining and Technology (NMIMT). The EMRTC facility has a 60 year history of tens of thousands of accident-free tests involving high explosives, rocket motors, ballistic projectiles, rocket sleds, and other high-energy phenomena. The liquid sodium tests will be performed by remote operation in a safety test area that is dedicated to the sole use by the Dynamo Project Group (DPG). Compared to high-explosives (HE), sodium is very safe because it cannot detonate. One of the DPG members, S. A. Colgate6 has had extensive hands-on use of liquid sodium in laboratory experiments for determining MHD stability for fusion confinement. 1. Receipt of the Sodium MetalThe Energetic Materials Research and Testing Center (EMRTC) facility at the New Mexico Institute of Mining and Technology (NMIMT) will act as the receiving agent of the solid sodium metal when shipped from Dupont Specialty Chemicals Co., Niagara Falls, NY. This is so that the solid sodium material can be received by a controlled facility for energetic materials. The sodium metal will be shipped in a 55 gallon steel drum containing 330 lb of fused sodium (UN1A1/X100/S/USA).1 Drums of fused sodium offer convenient supply of intermediate volumes (max 420 lb).1 Volumetrically, 330 lb of sodium metal displaces ~ 41 gal.2 The shipping containers will be padded with nitrogen gas (an anti-oxidant), though inert padding with argon and helium is also available. The United States Department of Transportation (DOT) classification for sodium is "Dangerous When Wet". The DOT identification number for sodium metal is UN 1428. DPG will be available to manage, oversee, or assist with the receipt of the sodium metal as EMRTC sees necessary and fit. 2. Storage Facility RequirementsTHE SODIUM METAL MUST BE STORED IN A WEATHERPROOF BUILDING THAT DOES NOT HAVE A WATER FIRE EXTINGUISHER SPRINKLER SYSTEM. "Sodium should be stored in a dry, fireproof building in sealed metal containers under inert gas blanketing or under mineral oil. A remotely located, detached building is preferred. The storage space must be large enough to accommodate both the maximum expected inventory of sodium and any empty containers. The building should not contain flammables, combustibles, or water and should not have a sprinkler system, water pipes, steam pipes, or skylights. Particular care must be taken to prevent water entry from roof leaks, rain, snow, or improper drainage. Potential for flooding should be evaluated."1 Conforming to the above requirements for a sodium storage facility, EMRTC has provided the DPG with a small steel building that will be dedicated to sodium storage only. The building is made of 1/2" steel plate throughout the floor, walls and roof, with 2" channel used to displace the floor above the ground. Externally, it is 4' × 4' square, 7' tall at the rear and 7'6" tall at the front. The 7 deg slanted roof projects 20" out from the front. The door is 3'6" wide × 7' tall, and can readily be made completely weatherproof and secure. This building was chosen because it conveniently accommodates the sodium-filled drum/pallet system, discussed in Sec. 5 (Preparation for Long-term Sodium Storage), for long-term safe, weatherproof, accessible storage. For reasons discussed in Sec. 6 (Storage and Transfer System for Sodium Use), DPG prefers that the sodium is stored under a blanket of mineral oil which we will supply. DPG will be available to manage, oversee, or assist with the storage of the sodium metal as EMRTC sees necessary and fit. 3. HandlingIt is not anticipated that at any time sodium metal, solid or liquid, will be intentionally physically handled by any person associated with the project. 3.1. Solid Sodium SafetyAlthough DPG does not anticipate that any personal physical contact with the solid sodium metal will take place, safety issues dictate that all Safety Practices and Procedures Must be Absolutely Followed at All Times. The development of Safety Practices and Procedures for the Liquid Sodium Dynamo Experiment will conform to industry standards and be suited specific to this application. DPG and EMRTC are working together to develop and refine the Safety Practices and Procedures for the Liquid Sodium Dynamo Experiment. 3.1.1. Personal Protective EquipmentHandling solid sodium metal requires special Personal Protective Equipment (PPE) to avoid personal injuries. A variety of PPE will be made available by the DPG, and the Proper Equipment for the particular job or task Must Be Worn. PPE availability, training, planning, and working carefully are the keys to preventing serious injuries and/or fires involving sodium metal. A list of the PPE made available for solid sodium handling is as follows: chemical splash goggles, loose-fitting dry moleskin mittens, neoprene or NomexTM apron, approved hard hat, industrial safety shoes. 3.2. Liquid Sodium SafetyAlthough DPG does not anticipate that any personal physical contact with the liquid sodium metal will take place, safety issues dictate that all Safety Practices and Procedures Must be Absolutely Followed at All Times. The development of Safety Practices and Procedures for the Liquid Sodium Dynamo Experiment will conform to industry standards and be suited specific to this application. DPG and EMRTC are working together to develop and refine the Safety Practices and Procedures for the Liquid Sodium Dynamo Experiment. 3.2.1. Personal Protective EquipmentAdditional protective equipment including fire retardant clothing is required when handling liquid sodium metal. It is essential to layer and drape the clothing to optimize its protection, so a variety of clothing should be worn. Flame retardant clothing made from 6.0 Oz NomexTM fabric (air permeability of less than 100 ft/min) will be made available by DPG. The clothing will be designed so that it is loose fitting, and can be readily torn or cut off quickly in case the wearer is splashed with liquid sodium. Leather belts should not be worn. Heavy leather safety shoes with leather or canvas spats attached, can be worn for additional protection. A face shield must be worn in addition to goggles when protection of the entire face is needed. A face shield is not a replacement for chemical goggles; they must be worn together when a face shield is required. Special chemical splash goggles are recommended and will be provided by DPG; American Optical Special Goggle No. SCS247-711, with cemented-in 0.060" lenses, rubber band, and special forehead and nose shields. The nose shield is equipped with a short hook on the beak which permits effective use of a fire- retardant NomexTM aramid fiber bandanna to cover the face, ears and neck. Use of this goggle/bandanna offers excellent head protection while maintaining comfort and good communication capability. Mole skin mittens will be used for hand protection. Mittens can be slipped off quickly if they are splashed with liquid sodium. 4. Fire Fighting"Sodium fires are extinguishable with dry sodium carbonate ( light soda ash) or by exclusion of air. The following materials should not be used on sodium fires, since they react violently with sodium (see "Chemical Properties"):
Class A, B, and C fire extinguishers should never be used on sodium fires and normally should not even be stored in the sodium area. Class D or dry powder extinguishers containing soda ash, Na-XTM or Metl-XTM are compatible with sodium, provided nitrogen and not carbon dioxide is used to expel the powder. Caution should be used with a pressurized extinguisher because the liquid sodium may be spattered by the discharge from the extinguisher. Salt or dry graphite powder may also be used on sodium fires, but they are less effective than dry soda ash. Signs should be posted at building entrances and throughout the area warning against the use of water and standard type fire extinguishers."1 "Plant and local fire departments that might respond to a fire in a sodium area should be afforded prior and periodic training specific to sodium use areas and fire fighting techniques. These personnel should know where sodium is stored and acceptable techniques to extinguish sodium fires. Fire response personnel should consult knowledgeable plant personnel in determining proper sodium fire fighting actions."1 "Sodium fires burn on the surface of a molten sodium "pool." Fires may be extinguished by smothering, after first containing the liquid sodium to minimize its surface area. Firefighters should stay upwind. Dry soda ash can be used to dike a sodium spill, and liberal quantities should be spread over the burning surface. If sodium is burning in open equipment such as a drum or tank, sheet steel can be used to cover and smother the fire."1 5. Contingency for Liquid Sodium Spill and CleanupThe DPG recognizes the need to be prepared for the unlikely, but possible event that a spill of liquid sodium metal could occur. During the operation of the experiment the apparatus is surrounded by a safety shield that is designed to contain the loss of sodium should a mechanical failure occur: See Sec. 8.4, Safety Shield for the Apparatus. It is necessary to note that either small quantities or large, thin distributions of liquid sodium will cool to the solidification temperature very rapidly. Thus the cleanup of spilled liquid sodium material would most likely have to deal with a solid sodium metal distribution. In preparation for any unexpected equipment failure during transfer of liquid sodium metal that could result in a large-scale, catastrophic loss of liquid sodium metal or mineral oil from the apparatus or drum, a number of buckets of dry, light soda ash (Na2CO3) to dike and cover the sodium metal will be available in the immediate vicinity of the experiment. The action of covering the liquid sodium metal with Na2CO3 will help to exclude the oxidizing atmosphere from the surface and abate the possibility of a sodium fire. Should a sodium fire occur, the Na2CO3 would be used to cover or smother it, and extinguish the sodium fire by the exclusion of air.1 In preparation for these contingencies, the following materials and equipment will be available at the test site (in addition to all aforementioned PPE):
During the process of transferring liquid sodium metal from storage drum to apparatus and apparatus to storage drum, local and EMRTC fire department personnel and their equipment will be on hand to assist in an emergency resulting from a loss of sodium metal. The operation of the dynamo when filled with sodium will be done remotely: See Sec. 8, The Experiment. Should a dynamic loss of sodium from the apparatus occur, the safety shield is designed to contain it from being sprayed into the atmosphere and autocatalytically igniting. Should a fire result during the remote operation of the experiment, it has been mutually decided by DPG and EMRTC that the fire will be allowed to burn, and the smoke dissipate into the atmosphere. The building in which the experiment itself is run is considered to be disposable in this regard. In the unfortunate circumstance of a consuming fire starting, the loss of the experimental apparatus is of far greater impact than the building in which its operated. 6. Preparation for Long-term Sodium StorageWhen the solid sodium metal is initially received at the specified EMRTC facility for storage, the drum containing the fused sodium will be prepared to have the preponderance of the nitrogen gas padding replaced with a blanket of mineral oil (white oil) for long-term storage stability and ultimate use. The drum in which the fused sodium is shipped has a 2" and a 3/4" bung on the top and a 2" bung on the bottom in line with the 2" top bung. All three of these bungs have standard NPT threads. Initially the drum will be opened at the 3/4" bung and have a tee fitting connected to it such that an external supply of nitrogen gas can be introduced and maintained inside the drum at the top through one port of the tee while a pressure control valve is fitted in the second port. The pressure control valve will be set at a maximum of one ounce per square inch pressure as a safety measure because the drums are not built to be pressurized. Prior to filling the drum with the mineral oil blanket, the drum will be lifted onto its own custom-made, fitted-pallet that accommodates the gravity-feed drain ball-valve that is to be permanently installed in the bottom 2" bung. Under the maintained head-pressure of nitrogen gas at one ounce per square inch, the bottom 2" bung will be removed and replaced with an elbow fitting and the ball valve. Subsequently the mineral oil will be introduced to the drum through the 2" top bung while the nitrogen gas padding is vented through the pressure control valve. This will set up the drum/pallet system containing the fused sodium for long-term storage and use. 7. Storage and Transfer System for Sodium UseTo use the sodium metal in the experiment, the drum/pallet will need to moved from the storage building to the experiment test area. The use of the sodium metal in the drum will take place by heating the drum to liquefying temperature, 110 C (230 F), and gravity-feed transferring the liquid sodium to the preheated experimental apparatus through heated fluid transfer lines.7 A single recirculating hot-oil heat source will be used to liquefy the sodium in the drum and in the apparatus. DPG will be using a standard 20 kW electric hot water heater filled with mineral oil as the heat source. The sodium metal storage drum will have approximately 100' of 3/4" soft copper tubing wrapped around it in a coil consisting of approximately 16 turns. A high-quality insulating blanket will encase the coil-wrapped drum assembly on its pallet. A 2 L/s (1/2 gal/s) hot-oil pump set up in a recirculating configuration to heat both the drum and the apparatus will connect to the drum's coils and the apparatus through heated valves and low-flow-restriction detachable connections. Figure 1 shows a plan-view schematic of the equipment configuration stated above. Figure 2 shows a side-view schematic of the equipment configuration stated above where the drum/pallet system is raised to allow the gravity-feed transfer of the liquid sodium metal from the drum to the apparatus. Given the various thermal characteristics of heat transfer for the system, calculations show that liquefying the sodium will take approximately 30 minutes. In addition we will have two electric heat guns available during transfer operations along with a thermocouple probe with a remote readout of temperature so that any unplanned solidification of sodium during transfer can be readily identified and rectified.
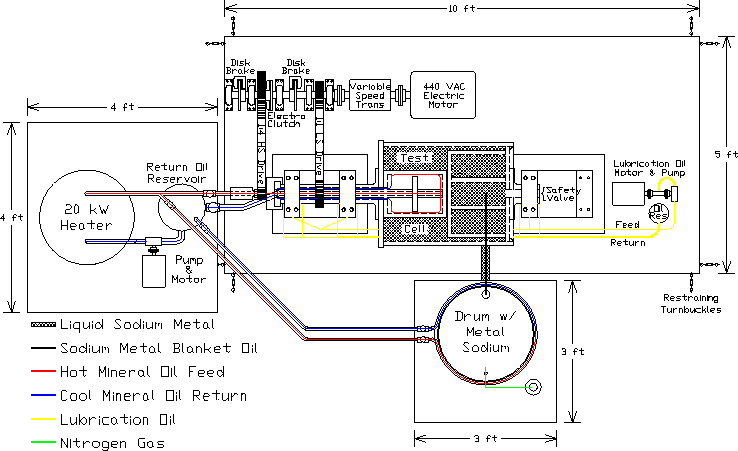
Figure 1: A plan-view schematic of the Ω-Phase of the Liquid Sodium α ω Dynamo experiment apparatus and sub-system equipment. The main platen supports the test cell apparatus on its bearing pedestals, the electric motor drive system and the lubrication oil system. The motor drive system as shown consists of a continuous-duty AC motor, variable speed transmission, and main drive shaft. The main drive shaft is a pillow-block bearing supported shaft on which are mounted the low-speed (LS) 1:1 drive sheave, the electro clutch that engages to drive the high speed (HS) 1:4 drive sheave, and 2 electric actuated disk brakes. The electric disk brakes are configured such that the primary disk brake is engaged when energized to slow or stop the rotation as desired. The secondary disk brake is configured to be engaged when de-energized for emergency shut-down purposes. The necessity of this is such that if something happens and the experiment needs to be immediately and completely shut down, the secondary brake will automatically engage by de-energization (killing the power) and stop all rotation. The heating equipment consists of a 20 kW electric heater, hot-oil pump and electric motor, return oil reservoir, control valves and low-flow-restriction detachable fluid transfer connections. The safety shield that surrounds the rotating test cell apparatus is removed for the sodium transfer process, and is therefore not shown. There are several points to note about the the use of sodium metal and mineral oil in the storage drum/pallet system and the experimental apparatus.
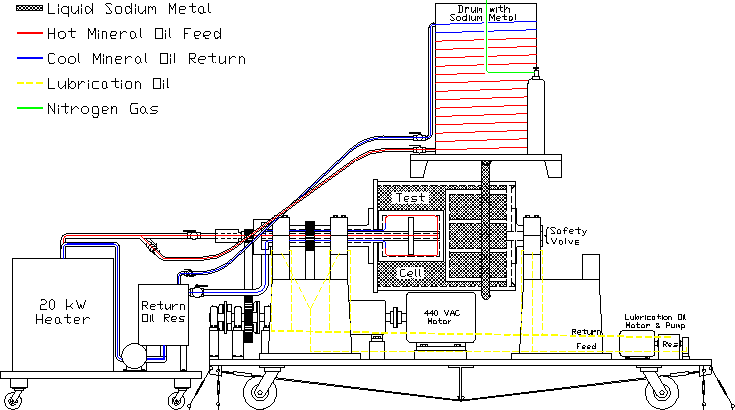
Figure 2: A side-view schematic of the Ω-Phase of the Liquid Sodium α - Ω Dynamo experiment apparatus and sub-system equipment. Note that the drum/pallet system is raised to allow the gravity-feed transfer of the liquid sodium metal from the drum to the apparatus. The electric forklift used to raise the drum/pallet system is not shown for clarity. 8. The ExperimentFigure 3 shows some of the constructed parts the Ω-Phase of the Liquid Sodium α - Ω Dynamo experiment. The main plate upon which the constructed assembly resides is 5 feet wide × 10 feet long. The motor for rotational power and the power transmission system is to be mounted on this platen axially parallel to the dynamo apparatus. An independent pressurized oil system for the primary bearing lubrication will also be mounted on this platen. The separate heat-source system discussed above will need to be placed in line with, and at the end of the platen at what is the right-hand side in Fig. 3. The approximate size of the heat-source system will be 4' × 4'. In order to transfer the sodium metal from the insulated storage drum/pallet system to the apparatus it will need to be placed along side the platen on what is the far side in Fig. 3, and raised to a height of ~ 5' from the floor. This is to facilitate the gravity-feed transfer of the liquefied sodium metal from the drum to the apparatus. The approximate size of the drum/pallet system will be 3' × 3'. Figure 1 shows a plan-view schematic of this equipment configuration. Figure 2 shows a side-view schematic of this equipment configuration where the drum is raised to facilitate the gravity-feed transfer of sodium to the apparatus. A capable electric forklift is probably best suited to the task of lifting the drum/pallet system. There are three facets of reasoning for using an electric forklift which are due to the approximate 1 hour of time required for heating and transferring the sodium metal. These facets are: 1) electric forklifts utilizing a ball-screw jack don't drift down with time like so many hydraulic forklifts, 2) electric forklifts don't present the problems associated with carbon monoxide output in an enclosed area, 3) electric forklifts are typically more maneuverable than equivalently capable engine-driven forklifts. Draining of the apparatus by gravity-feed transfer back to the drum can take place at floor level. Subsequent to transferring the liquid sodium metal from the drum to the apparatus, the drum will be removed from the immediate work area.
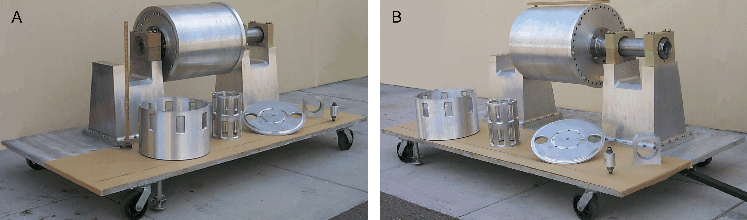
Figure 3: Figure 3 shows the constructed parts the Ω-Phase of the Liquid Sodium α - Ω Dynamo experiment mounted on the bearings and supporting pedestals. Also shown is the port plate and two ported reservoir plenum cylinders and the inlet & outlet recirculating oil rotary unions. The primary drive shaft, belt pulleys, and electric motor have yet to be added. As can be seen in Figs. 2 and 3, the dynamo apparatus is semi-portable in the sense that it is mounted on large casters and capable of being towed by a suitable piece of equipment over smooth surfaces. It will be taken to and removed from the EMRTC test facility by means of a 3-ton flatbed truck. The static weight of the complete experimental assembly is ~ 2.5 tons. When delivered to the site, a suitable forklift will be used to lift the apparatus from the truck. A series of screw-jacks will be fitted to the bottom of the platen to stabilize the apparatus for rotational tests. The main platen will be restrained to the floor of the test area by 8 turnbuckles situated as normal pairs at each corner (Figs. 1 and 2). The operation of the dynamo when filled with sodium will be done remotely. The apparatus and its diagnostic systems were intentionally designed to be remotely operated. The temperature, pressure, rotation rate and volumetric flow rate at a number of locations on the rotating equipment will be monitored by computer. In addition, the heating system and lubrication system will be provided with separate safety measures. Real-time monitoring within design-limit thresholds will allow for emergency shutdown of rotation and heating systems, while leaving the bearing lubrication system active. We will be using a large 1-ton utility van as the remote operations facility. This van has been provided by the NMIMT Research and Economic Development Division (R&ED). 8.1. Safety Valve for the ApparatusA pressure-relief safety valve is incorporated into the apparatus to relieve any pressure buildup from heating or from a transient pressure spike. The safety valve will mechanically exhaust material from the apparatus test-cell into a containment vessel which is at atmospheric pressure. It will operate automatically to relieve pressure at a preset value. It is designed to be capable of operating over thermal range of 20 - 150 C. The safety valve is physically situated on the rotation axis of the apparatus. It is incorporated into, but not integral with the thrust-end shaft of the apparatus (Figs. 1 and 2). 8.2. Safety Shield for the ApparatusA removable, electrically heated, safety shield will surround the cylindrical vessel and will be used whenever the apparatus is rotated and filled with either mineral oil or sodium. The safety shield is designed to confine any dynamic sodium metal loss and absorb the kinetic energy and containment of disrupted parts should a mechanical failure occur. The safety shield also provides a 115 deg C thermal region immediately external to the vessel so that the thermal load and gradient in the experiment is reduced. 8.3. Space RequirementsThe experimental apparatus and its related systems will take up a rectilinear area of ~ 10' wide × 15' long (Fig. 2). There will be a minimum of a 5' wide clear work/walk space around this area. Thus an approximate minimum area of 20' in width and 25' in length will be used for the safe setup and operation of the dynamo. 8.4. Power RequirementsThe electrical power for the rotation of the dynamo will be provided by a 3-phase, 440 VAC, 50 amp circuit. Rotational power can be provided by either a continuous-duty AC motor that drives a variable-speed transmission or an AC-motor/DC-generator set that drives a DC motor for variable speed. We are flexible in this sense because we have not yet selected the rotational power source. The 20 kW electric hot-oil heat system will be run from a 220 VAC single-phase power source. This is intended be a standard residential-type "hot water" heater. This system will also include the use of a 3 kW hot-oil recirculation pump as described above, also run by single-phase 220 VAC. The lubrication system will run on standard 110 VAC, 20 amp service. The remote operation van will need to be serviced with a minimum of two 110 VAC lines for operations of the remote controls, alarms, computer, lights, etc. The remote operations van can be displaced up 200 meters away from the experimental operation site as safety dictates and warrants. 9. References1 Dupont Specialty Chemicals, Technical Information, E-92775-1 (1994) 2 The volumetric displacement of the Liquid Sodium α ω Dynamo Experiment in its Phase-I configuration is 155.6 L = 1.556 × 105 cm3. Sodium metal has a solid density at 20 C of 0.968 g/cm3, therefore the mass of sodium that the apparatus can contain is 150.6 kg = 332 lb. 3 Gailitis, A., Lielausis, O., Dement'ev, S., Platacis, E., Cifersons, A., et. al. "Detection of a Flow Induced Magnetic Field Eigenmode in the Riga Dynamo Facility" Phys. Rev. Let. 84 4365-4368 (2000). 4 Busse, F.H., Müller, U., Stieglitz, R., & Tilgner, A., "A Two-scale Homogeneous Dynamo: An Extended Analytical Model and Experimental Demonstration Under Development", Magnitnaya Girdrodinamika, T.32, 259-271 (1996). 5 Raedler, K.-H., Apstein, E., Rheinhardt, M., and Schüler, M., "The Karlsruhe Dynamo Experiment; A Mean Field Approach" Studia geoph. et geod. (Prague) 42 1-9 (1998). 6 Colgate, S.A., "Liquid-sodium Instability Experiment", UCRL 4560 (1955). 7 No transfer of sodium from the drum to apparatus or apparatus to drum will take place using pumps. The safety reason for this is that even when heated, the thermal conductivity of the sodium is great enough that a localized solidification can develop through heat-transfer cooling and the pump can dead-head, cavitate or freeze shut, depending on the location of the solidification, and can ruin the pump, burn up the motor, or break fluid transfer lines. 8 The dynamic radial seals used in the apparatus have as the lip material the fluoroelastomer FKM (ASTM D 1418 symbol), being the same as FPM (ISO 1629 symbol). This material offers the greatest chemical resistance to mineral oil, sodium metal and its derivatives (NaOH and Na2O2) of any conventionally used lip seal material. The fluoroelastomer LongLifeTM seals from Chicago Rawhide are made of the premium lip-seal material that has the widest temperature range and chemical resistance. The LongLifeTM seals will handle temperatures from -40 to 204 C (-40 to 400 F). They resist most special lubricants and chemicals that can destroy nitrile, polyacrylates or silicons. Even though the fluoropolymer PTFE offers wider media resistance in comparison to standard elastomers with a temperature range of -73 to 260 C (-100 to 500 F), it is not to be used for the seals in the experiment because sodium metal is one of the few materials that will adhere to PTFE and mechanically degrade its sealing ability. 9 Tests in the NMIMT Dynamo Lab with a 3 cm i.d. cylinder have shown that the "wetting" of the aluminum surface with mineral oil does completely nullify any chemical interaction between sodium metal and aluminum. These tests were performed at 115 C in both static and rotating configurations, where the centripetal acceleration was ~ 54 g, and the duration of the test was 1 day. Under these conditions the mineral oil was centrifugally spun to the center of the cylinder because it represented the low-pressure, or buoyant region of the test apparatus. This is simply due to the fact that the mineral oil has a lower specific gravity than that of the liquid sodium: ~ 0.81 vs ~ 0.93 at 100 C. Even though the mineral oil was centrifugally spun out of the interface region to very thin dimensions (as measured by electrical resistivity), no reaction took place between the sodium and aluminum because there was no atmospheric oxygen or moisture able to enter the interface region. The conclusions from the DPG tests are that without O2 present to form Na2O2 or H2O to from NaOH at the interface, no chemical reaction between the liquid sodium metal and aluminum could take place. About this document ...This document was generated using the LaTeX2HTML translator Version 97.1 (release) (July 13th, 1997) Copyright © 1993, 1994, 1995, 1996, 1997, Nikos Drakos, Computer Based Learning Unit, University of Leeds.
The translation was initiated by Kate Weatherall on 11/22/2000
|