Alpha Particle Spectrometry with
Surface-Barrier Detectors


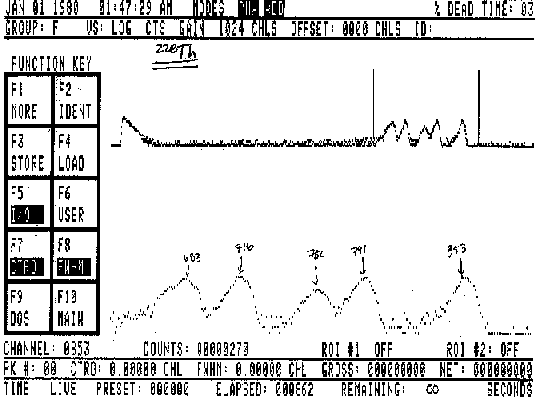
Heavy radioactive elements emit alpha particles at discrete energy values. In this experiment, we record this spectrum of alpha particles from a radioactive sample at different air pressures. Then, plotting the energies (E) versus air pressure (P), we graphically differentiate this to find dE/dP, and finally graph dE/dP versus E, which should show an inverse relationship. First the spectrometry system is set up and voltage pulses are observed on an oscilloscope. These correspond to alpha particle collisions with the detector and subsequent amplification of the detector output. A multi-channel spectral analyzer interfaced with a PC is calibrated using the known decay energy of 230Th.
The experiment requires several spectra at varying air pressures to get a graph of of alpha particle energy versus pressure, still using 230Th. Once this is accomplished we can graphically differentiate the curve to get dE/dP and a graph of dE/dP versus energy.
Another objective of the experiment is to identify unknown radioactive
samples by measuring the energy spectrum of emitted alpha particles.
The 230Th sample is replaced with the unknown sample and
a spectrum at low pressure is obtained. From this the student should
be able to identify the isotopes present in the unknown.